Abstract
Background: Acute myeloid leukemia (AML) cells are sensitive to depletion of extracellular glutamine (Gln) or manipulation of Gln metabolism. We recently reported the synergistic anti-leukemic activity of the combination of the Bcl-2 inhibitor Venetoclax (Ven) and pegcrisantaspase (PegC), a pegylated Erwinia asparaginase, in complex karyotype AML (CK-AML). The potent in vitro and in vivo activity of Ven-PegC regimen is mediated through inhibition of 4EBP1 phosphorylation resulting in decreased mRNA translation and protein synthesis. We hypothesized that safe and tolerable doses of Ven-PegC will result in anti-AML activity in patients (pts) with relapsed/refractory (R/R) AML.
Study Design and Methods: The study is an open-label phase 1b clinical trial (NCT04666649) with 3+3 design with 4 cohorts of Ven (400 mg daily in the cohorts 1-3, 600 mg daily in cohort 4) in combination with outpatient intravenous PegC (500, 750, 1000, 1000 IU/m2 in cohorts 1-4, respectively) biweekly in a 28-day cycle. Key eligibility criteria include AML in an adult that has relapsed after, or is refractory to first-line therapy including Ven-containing regimens. The primary endpoints are the incidences of regimen limiting toxicities (RLT) and treatment emergent adverse events of Ven-PegC. Secondary endpoints include rate of composite complete remissions. Exploratory endpoints include the evaluation of plasma amino acid levels of Gln, asparagine (Asn), glutamate (Glu), and serine (Ser), and plasma asparaginase activity post-PegC administration.
Results: As of August 1, 2022, 11 pts who received at least 1 dose of PegC were enrolled into the first two cohorts. The median age was 60 (range 24-76) years; 6 (55%) were female; 10 (91%) had adverse-risk AML by European Leukemia Net (ELN) criteria; 7 (64%) had CK-AML, and median prior treatments were 2.
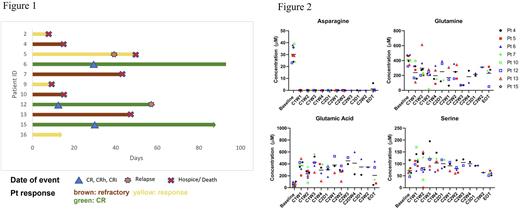
Overall response rate was 64% (7 pts) including 3 pts (27%) with CR/CRh/CRi [2 previously Ven-refractory]. In the first cohort, pt2 cleared peripheral blood (PB) blasts within 3 days after the first dose of PegC. Pt5 bone marrow (BM) blasts significantly decreased (78% to 9%) after cycle 1, and pt6 achieved CR with partial hematologic recovery (CRh) with MRD-negativity after 1 cycle and proceeded to alloHSCT after 3 cycles of Ven-PegC. In cohort 2, pt9 cleared PB blasts within 3 days after the first dose of PegC, and pt12 (primary refractory AML with two TP53 mutations) achieved a rapid clearance of PB blasts (41% to 0% after 2 days) and BM blasts after 1 dose of PegC and achieved MRD-negative CR at the end of cycle 1. Pt15 achieved CR with incomplete hematologic recovery (CRi) after 1 cycle of Ven-PegC and is currently receiving cycle 3, and in pt16 absolute PB blasts reduced from 4876/mcL to 192/mcL on Cycle 1 Day 3 and is currently receiving cycle 1 (Fig 1).
Eight patients were evaluable for RLT assessment. No RLT was observed in cohort 1. In cohort 2, one transient (<48h) asymptomatic hypertriglyceridemia was observed and resulted in the expansion of cohort 2 to 6 patients. No serious asparaginase-related adverse events of interest have been observed.
Pharmacokinetic and pharmacodynamic data are available for 8 patients. All pts achieved a nadir plasma asparaginase activity >0.1 IU/mL during cycle 1 and repetitive doses of PegC did not result in silent inactivation. Mean (range) plasma amino acid levels (µmol/L) at baseline were: Asn 31 (24-38), Gln 412 (323-465), Glu 58 (22-103). All pts achieved undetectable Asn level by day 7 and Asn remained undetectable throughout the study. Plasma Gln reduction from baseline occurred in all pts within cycle 1, with pts in cohort 2 demonstrating a more rapid decrease in Gln levels after initial exposure, confirming a dose-response correlation. Of note, in both cohorts, the pts that responded to Ven-PegC achieved the lowest absolute plasma Gln levels. A concomitant increase in plasma Glu levels was observed in all pts, indicative of the PegC-induced conversion of Gln to Glu. Remarkably, plasma Ser increased ~2-fold at the nadir of plasma Gln, from 75 ± 21 µmol/L at baseline to 114 ± 30 µmol/L after the first dose of PegC (Fig 2).
Conclusion: Ven-PegC is a well-tolerated regimen with a novel metabolically-targeted mechanism of action. Early efficacy results in patients with poor prognosis R/R AML, a population with very limited treatment options, has been very promising. A clear correlation between the dose of PegC administered and the reduction in plasma Gln was observed.
Disclosures
Baer:Takeda: Research Funding; Forma: Research Funding; Kite, a Gilead Company: Research Funding; AbbVie: Research Funding; Kura Oncology: Research Funding; Ascentage: Research Funding. Emadi:Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy; Genentech: Membership on an entity's Board of Directors or advisory committees; KinaRx, Inc.: Other: Founder and Scientific Advisor; NewLink Genetics: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Research Funding.
OffLabel Disclosure:
Pegcrisantaspase for AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal